Activating the Caretakers
Astrocytes are considered the natural caretaker cells of the brain and are the most abundant cell type in the human brain 1,2. Astrocytes provide metabolic and trophic support to neurons and they modulate synaptic activity 3. After brain injury, astrocytes play pivotal roles in restoring ion balance, controlling edema, and removing toxic neurotransmitters 4-6. These neuroprotective activities are highly energy-dependent and require astrocyte mitochondria 7,8. Notably, neurons are permanently injured after ischemia only if astrocyte mitochondrial function fails 9,10. Inhibition of astrocyte mitochondria increases cell swelling and induces cell death11. Excitotoxicity, due to high extracellular glutamate, is also principally controlled by astrocytes and requires membrane polarization – the energy-dependent maintenance of the Na+ ion gradient12-14.
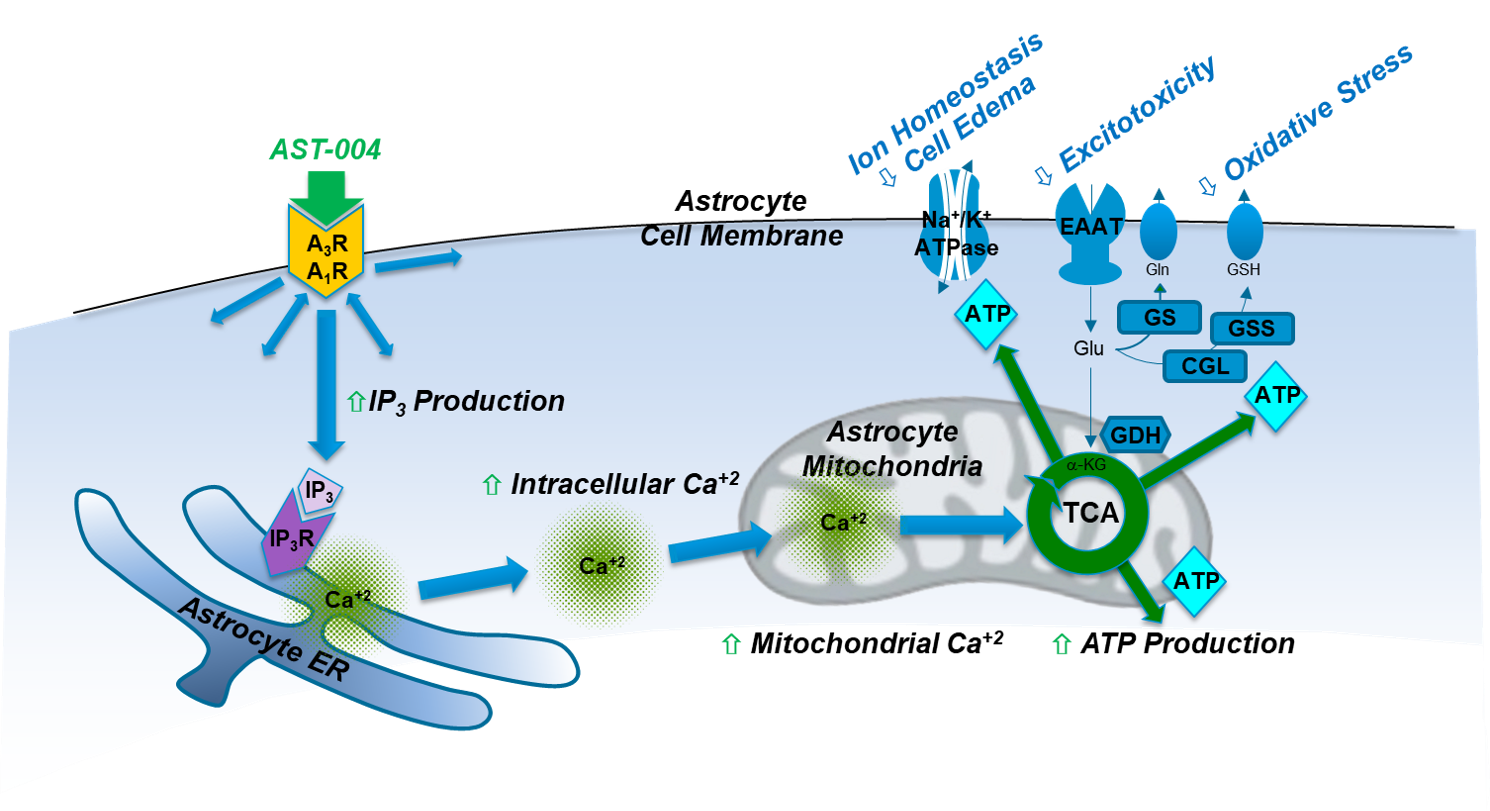
Adenosine is a ubiquitous endogenous compound that plays critical roles in nearly all aspects of cellular physiology throughout the body. Under normal physiological conditions, adenosine concentrations in the body are maintained at low nanomolar concentrations. However, under conditions of oxidative stress and trauma, dramatic increases in adenosine concentrations can be observed, leading researchers to classify the compound not as a hormone, but as a “retaliatory metabolite” with critical roles in maintaining cellular function. Extracellular increases in adenosine activate the adenosine A1 and A3 receptors on glial cells. On astrocytes, this produces inositol (1,4,5) triphosphate (IP3) that triggers intracellular Ca2+ release from thapsigargin-sensitive stores in the endoplasmic reticulum (ER), 15-18. During astrocyte activation, mitochondria, in turn, sequester some of the released Ca2+ and utilize it to activate Ca2+ sensitive dehydrogenases in the tricarboxylic citric acid cycle (TCA). This signaling cascade thereby increases oxidative phosphorylation and ultimately, significant production of ATP19-21.
Our results demonstrate that in multiple preclinical models of stroke and blunt trauma TBI, treatment with AST-004 significantly reduces early brain damage 17,18,22-26. These agonists selectively enhance mitochondrial ATP production in astrocytes by stimulating IP3-mediated Ca2+ release, which, in turn, enhance their energy-dependent neuroprotective functions. Pharmacological and genetic manipulation show that receptor activation not only increases neuronal and astrocyte survival but also partially reverses neuronal and glial damage. Our data also show that the protective pathway activated by our therapeutic candidates is conserved in ex vivo human brain tissue experiments (unpublished), emphasizing the potential for this novel approach to be an effective therapy for human brain injuries.
- Cherniak, C. The bounded brain: toward quantitative neuroanatomy. J Cogn Neurosci 2, 58-68 (1990).
- Nedergaard, M., Ransom, B. & Goldman, S.A. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 26, 523-30 (2003).
- Chen, Y. & Swanson, R.A. Astrocytes and brain injury. J Cereb Blood Flow Metab 23, 137-49 (2003).
- Nedergaard, M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 263, 1768-71 (1994).
- Parpura, V. et al. Glutamate-mediated astrocyte-neuron signalling. Nature 369, 744-7 (1994).
- Haydon, P.G. GLIA: listening and talking to the synapse. Nat Rev Neurosci 2, 185-93 (2001).
- Hertz, L., Peng, L. & Dienel, G.A. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab 27, 219-49 (2007).
- Watts, L.T. & Lechleiter, J.D. The impact of astrocyte mitochondrial metabolism on neuroprotection during aging. in Astrocytes in (patho) physiology of the nervous system (eds. Parpura, V. & Haydon, P.G.) 569-590 (Springer, Boston, MA, 2008).
- Largo, C., Cuevas, P. & Herreras, O. Is glia disfunction the initial cause of neuronal death in ischemic penumbra? Neurol Res 18, 445-8 (1996).
- Largo, C., Cuevas, P., Somjen, G.G., Martin del Rio, R. & Herreras, O. The effect of depressing glial function in rat brain in situ on ion homeostasis, synaptic transmission, and neuron survival. J Neurosci 16, 1219-29 (1996).
- Chu, X. et al. Oncosis, the possible cell death pathway in astrocytes after focal cerebral ischemia. Brain Res 1149, 157-64 (2007).
- Sonnewald, U., Westergaard, N. & Schousboe, A. Glutamate transport and metabolism in astrocytes. Glia 21, 56-63 (1997).
- Chatton, J.Y., Marquet, P. & Magistretti, P.J. A quantitative analysis of L-glutamate-regulated Na+ dynamics in mouse cortical astrocytes: implications for cellular bioenergetics. Eur J Neurosci 12, 3843-53 (2000).
- Oliet, S.H., Piet, R. & Poulain, D.A. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science 292, 923-6 (2001).
- James, G. & Butt, A.M. P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system. Eur J Pharmacol 447, 247-60 (2002).
- Verkhratsky, A. & Kettenmann, H. Calcium signalling in glial cells. Trends Neurosci 19, 346-52 (1996).
- Zheng, W., Talley Watts, L., Holstein, D.M., Wewer, J. & Lechleiter, J.D. P2Y1R-initiated, IP3R-dependent stimulation of astrocyte mitochondrial metabolism reduces and partially reverses ischemic neuronal damage in mouse. J Cereb Blood Flow Metab 33, 600-11 (2013).
- Talley Watts, L. et al. Purinergic 2Y(1) receptor stimulation decreases cerebral edema and reactive gliosis in a traumatic brain injury model. J Neurotrauma 30, 55-66 (2013).
- Denton, R.M. & McCormack, J.G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am. J. Physiol 259, E543-E554 (1985).
- Hajnoczky, G., Csordas, G., Krishnamurthy, R. & Szalai, G. Mitochondrial calcium signaling driven by the IP3 receptor. J Bioenerg Biomembr 32, 15-25 (2000).
- McCormack, J.G. & Denton, R.M. Signal transduction by intramitochondrial Ca2+ in mammalian energy metabolism. NIPS 9, 71-76 (1994).
- Zheng, W. et al. Purinergic receptor stimulation reduces cytotoxic edema and brain infarcts in mouse induced by photothrombosis by energizing glial mitochondria. PLoS One 5, e14401 (2010).
- Liston TE, Hinz S, Müller CE, et al. Nucleotide P2Y(1) receptor agonists are in vitro and in vivo prodrugs of A(1)/A(3) adenosine receptor agonists: implications for roles of P2Y(1) and A(1)/A(3) receptors in physiology and pathology. Purinergic Signal (2020).
- Bozdemir E, Vigil FA, Chun SH, et al. Neuroprotective Roles of the Adenosine A(3) Receptor Agonist AST-004 in Mouse Model of Traumatic Brain Injury. Neurotherapeutics. 18(4):2707-2721 (2021).
- Fisher ES, Chen Y, Sifuentes MM, et al. Adenosine A1R/A3R Agonist AST-004 Reduces Brain Infarction in Mouse and Rat Models of Acute Ischemic Stroke. Frontiers in Stroke (In Press, 2022).
- Liston TE, Hama A, Boltze J, et al. Adenosine A1R/A3R (Adenosine A1 and A3 Receptor) Agonist AST-004 Reduces Brain Infarction in a Nonhuman Primate Model of Stroke. Stroke. 53(1):238-248 (2022).