Reducing Damage in Stroke
AST-004 Efficacy in a Non-Human Primate Stroke Model
The significant efficacy of AST-004 in models of acute ischemic stroke (AIS) has been described in peer-reviewed reports published by Astrocyte Pharmaceuticals.
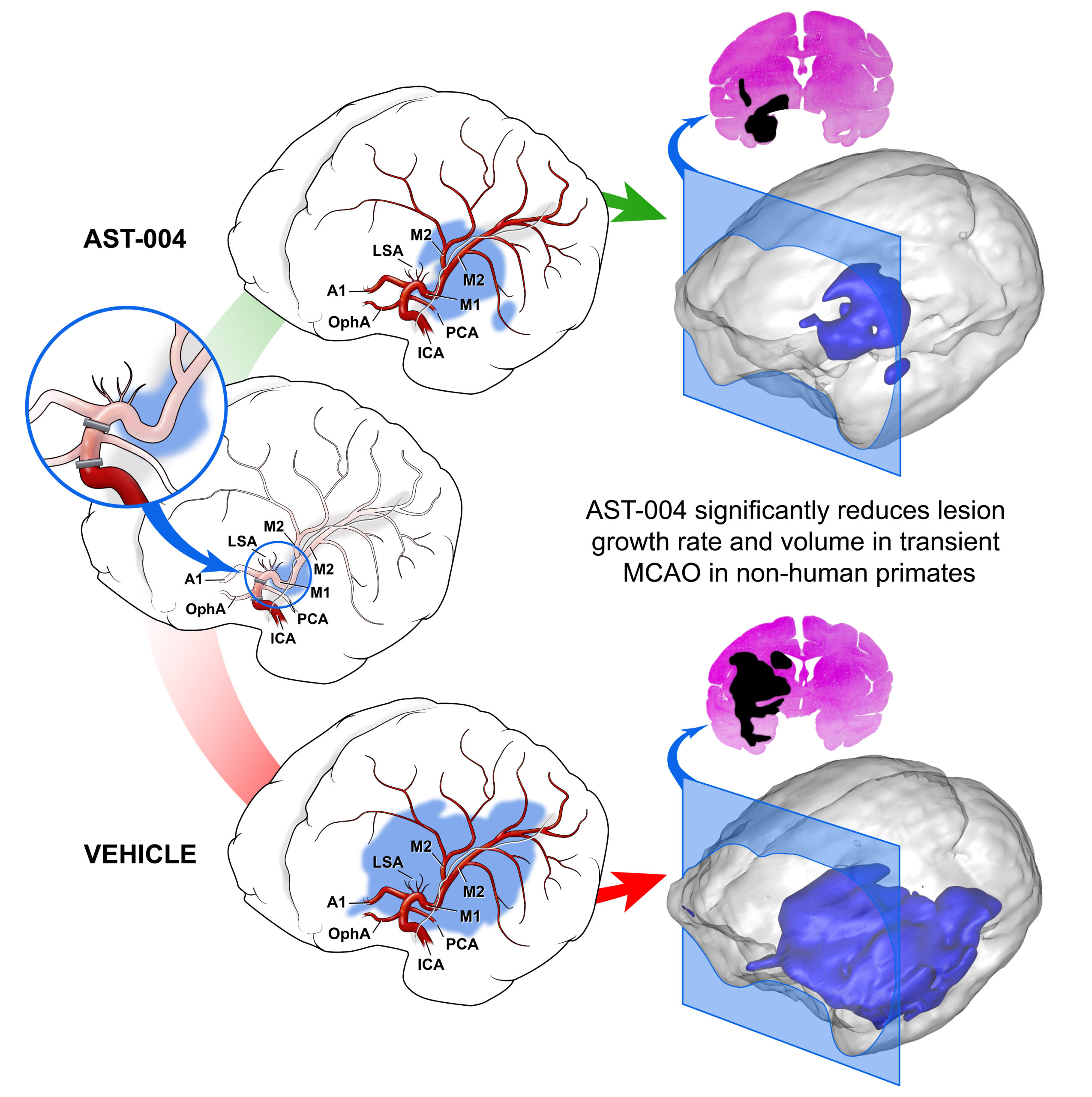
A key preclinical study, which was published in the journal Stroke (see Liston T. et al. 2022), employed clinical study intervention timelines in a nonhuman primate model of stroke. The animal models experienced a transient middle cerebral artery occlusion (tMCAO) starting at time zero, were treated with AST-004 (or negative vehicle control) with an intravenous bolus initiated 2 hours after the start of the tMCAO (and continuing with a 22-hour maintenance infusion), and were reperfused 4 hours after initiation of the stroke. This experimental timing is similar to recent stroke clinical trials (see Goyal M. et al, 2016) where human patients received alteplase intervention at 1.6 hours and reperfusion in 4-5 hours.
Primary outcome measures included lesion volume, lesion growth kinetics, penumbra volume as well as initial pharmacokinetic-pharmacodynamic relationships (PK/PD) measured up to five days after tMCAO. Secondary outcome measures included multiple physiological parameters such as heart rate, blood pressure and blood chemistry.
Administration of AST-004 resulted in rapid and statistically significant decreases in lesion growth rate and total lesion volume. In addition, penumbra volume decline over time was significantly less under AST-004 treatment compared to vehicle treatment. These changes correlated with unbound AST-004 concentrations in the plasma and cerebrospinal fluid as well as estimated brain A1R and A3R occupancy.
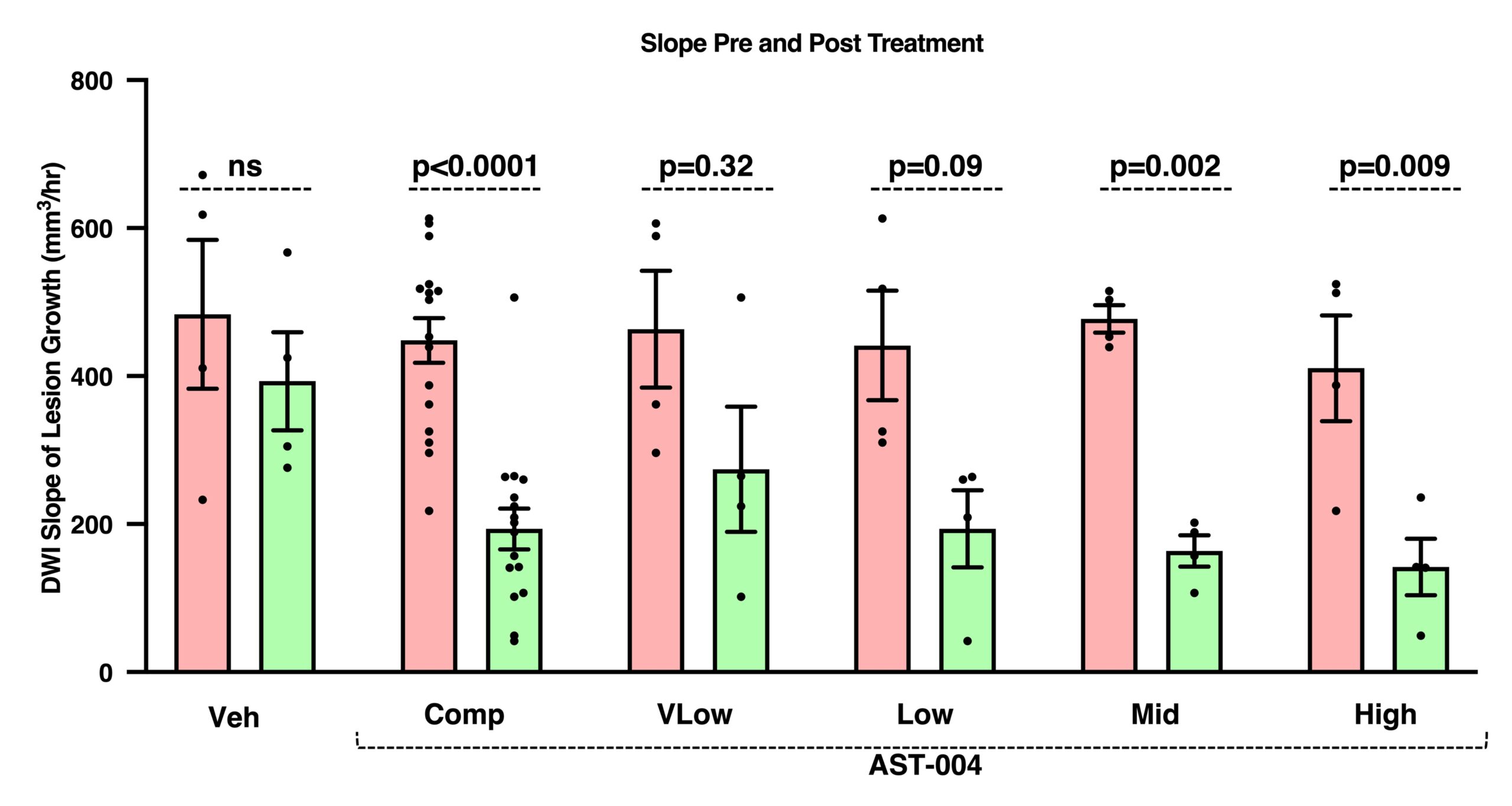
AST-004 Reduces Growth Rate of Stroke Lesion
Effect of AST-004 treatment initiation on the slope of DWI lesion growth following tMCAO in nonhuman primates.
Comparison of lesion growth slopes before and after initiation of vehicle or AST-004 treatment 2 hours post-occlusion. Results represent mean±SEM, n=4/dose group and n=16 for the composite (“Comp”) of all AST-004-treated groups combined. Pre-treatment slopes of lesion growth were determined for each subject from 0.5-1.8 hours post-occlusion. Initial post-treatment slopes were determined from 1.8-6.0 hours post-occlusion to provide sufficient timepoints for slope determination. Paired t-test comparing each group to itself before and after treatment.
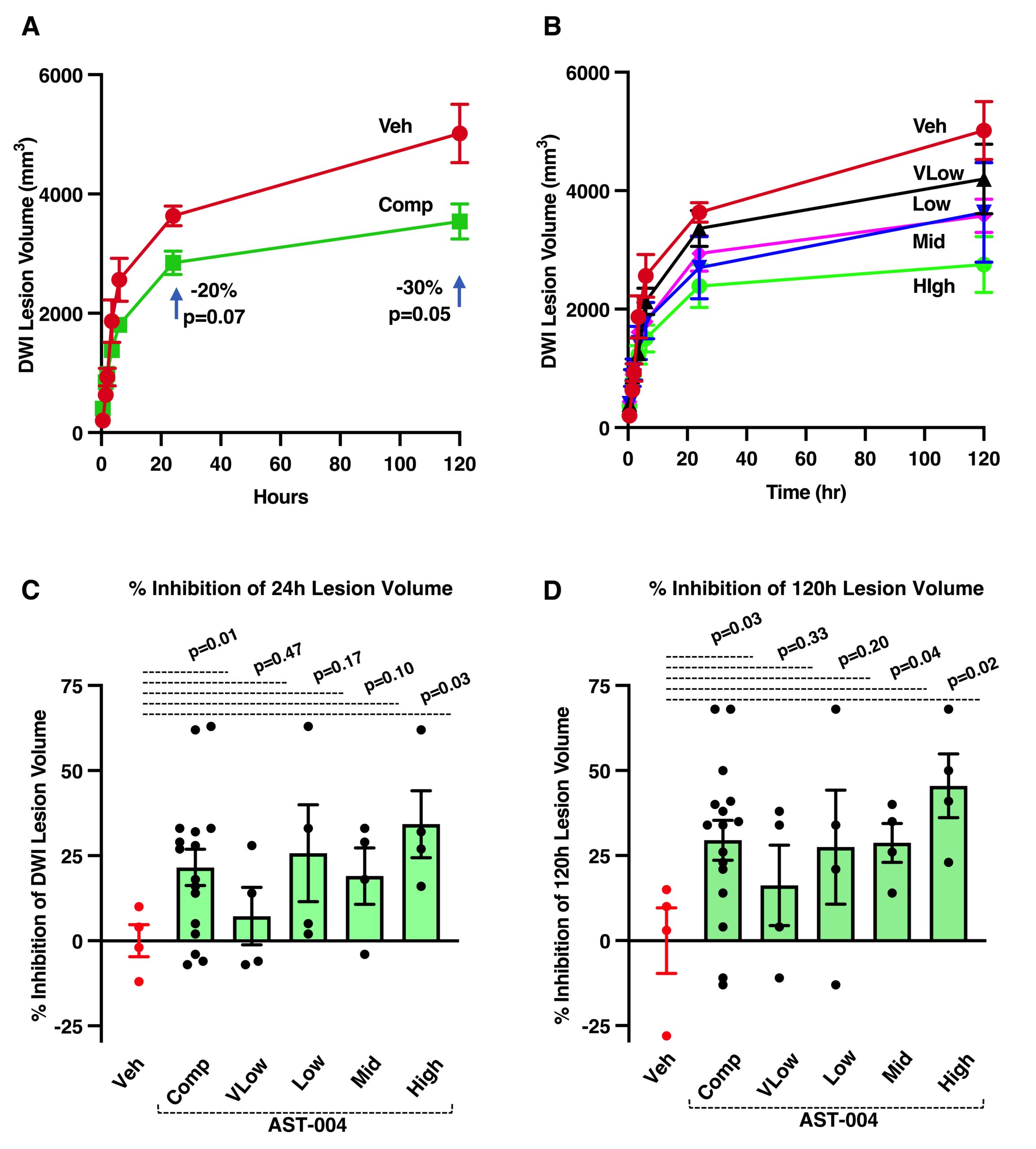
AST-004 Reduces the Volume of Stroke Lesion
Comparison of DWI lesion volume growth between vehicle- and AST-004-treated subjects following tMCAO.
(A) Comparison of DWI lesion size and growth between vehicle-treated and a composite of all AST-004-treated subjects. (B) Comparison of DWI lesion size and growth between vehicle-treated and each AST-004-treated dose group. (C) Comparison of percent inhibition of DWI lesion volume at 24 hours post-occlusion. (D) Comparison of percent inhibition of DWI lesion volume at 120 hours post-occlusion. Results represent mean±SEM, n=4/dose group and n=16 for the composite of all AST-004-treated groups. Unpaired t-tests.
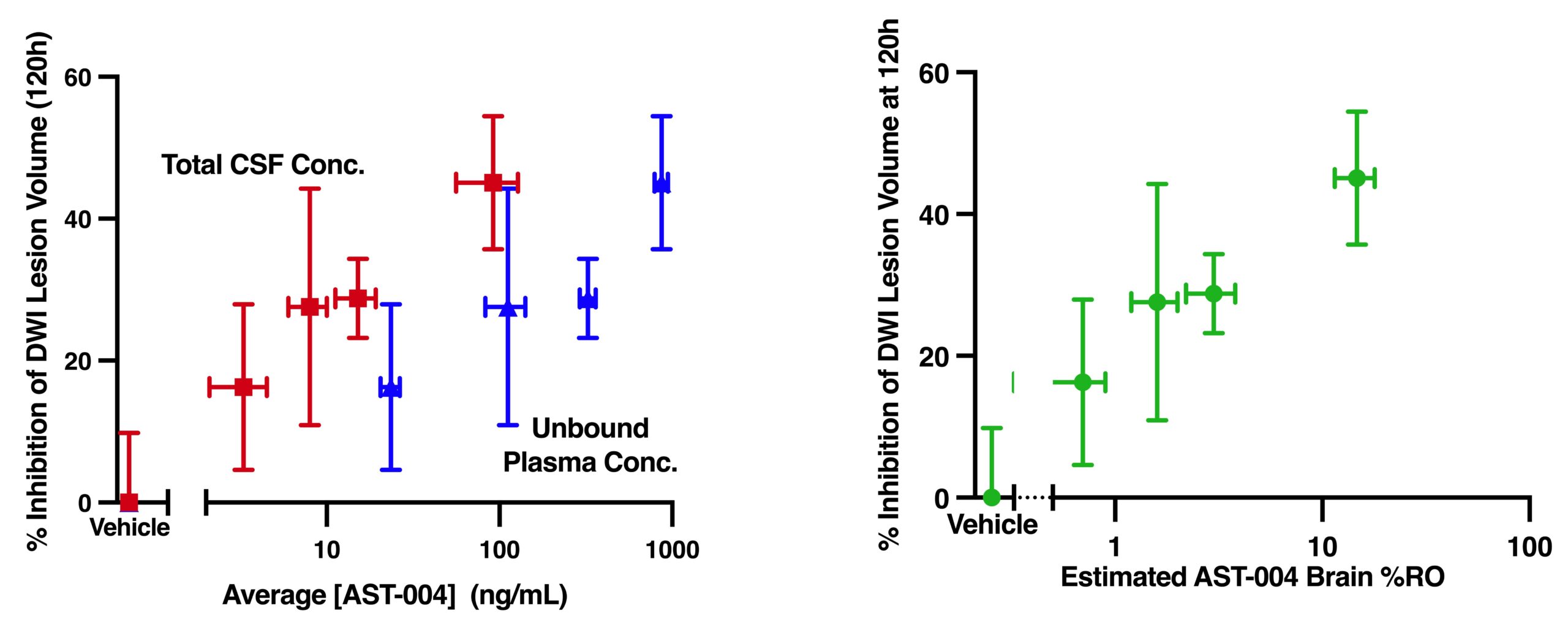
Cerebroprotective Effects Correlate with Unbound AST-004 Concentrations in the Plasma and CSF
Relationships between DWI lesion volume inhibition and average AST-004 unbound plasma concentrations, total cerebrospinal fluid (CSF) concentrations and estimated A1R/A3R brain receptor occupancy following MCAO.
Relationship between %inhibition of lesion volume at final DWI measurement (120h) and unbound plasma concentrations (red), total CSF concentrations (blue). Relationship between %inhibition of lesion volume at final DWI measurement and estimated AST-004 brain receptor occupancy of adenosine A1 and A3 receptors (green). Results represent mean±SEM, n=4/dose group.
For further detail, see:
Liston TE, et al. Adenosine A1R/A3R (Adenosine A1 and A3 Receptor) Agonist AST-004 Reduces Brain Infarction in a Nonhuman Primate Model of Stroke. Stroke. 2022; 53(1):238-248. PMID: 34802248.
Zheng W, et al. P2Y1R-initiated, IP3R-dependent stimulation of astrocyte mitochondrial metabolism reduces and partially reverses ischemic neuronal damage in mouse. J Cereb Blood Flow Metab. 2013; 33(4):600-11. PMID: 23321785.
Goyal M, et al. and HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016 Apr 23;387(10029):1723-31. PMID: 26898852.